PSI’s deep expertise in optical sensor innovation has found many applications in Life Sciences. Working closely with clinical physicians, we have developed and commercialized a family of laser-based optical instruments used for ophthalmic diagnostic applications. In this feature, we describe expanded applications of related technologies in imaging skin to guide cancer therapies and in brain tissue to assess drug uptake dynamics in neurons. In addition to clinical applications, we have developed and commercialized a group of laser-based optical sensors that are used to improve pharmaceutical manufacturing. In this issue, we include an update on the incorporation of these sensors into a comprehensive manufacturing software optimization tool that will be used by the pharmaceutical process development engineers for the production of advanced biologic drugs.
Biomedical Optics Technologies Group Highlights
Multimodal Optical Probe for Skin Cancer Diagnosis and Therapy Guidance
The Biomedical Optics Technologies Group (BOT) group, led by Dr. Nicusor Iftimia, has recently developed a second generation optical microscopy probe for diagnosis of skin lesions. Team members include Gopi Maguluri, software development; John, Grimble, mechanical design and fabrication; and Mircea Mujat, data optimization. The instrument will be used by the long-term collaborators at Memorial Sloan Kettering Cancer Center (MSKCC) to diagnose skin basal cell carcinomas (BCCs), assess their margins, and guide new non-surgical therapy modalities, such as radiotherapy, laser therapy, etc. Non-surgical treatments are emerging for superficial BCCs, superficial squamous cell carcinomas (incidence of ~ 1 million new cases/year in the U.S. alone), superficial lentigo malignas (incidence of > 40,000 cases/year in the U.S. alone), skin pre-cancers such as actinic keratoses (current prevalence is ~58 million in the U.S. alone), and superficial benign skin lesions such as solar lentigines, angiomas, sebaceous hyperplasias, and melasmas.
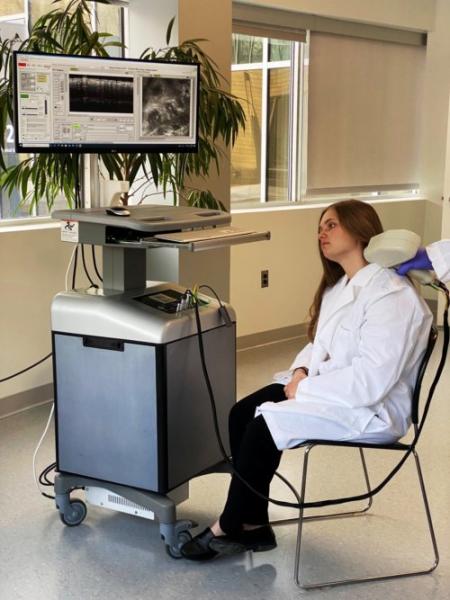
The PSI probe combines two optical modalities within the same optical layout: optical coherence tomography (OCT) and reflectance confocal microscopy (RCM). These two optical modalities complement each-other. RCM also provides submicron scale resolution, allowing for the identification of cellular-level morphological changes within the skin epidermal layer, such as cell palisading and collagen distortion. OCT has micron scale resolution and can resolve both the epidermal and the dermal layers of the skin, enabling assessment of lesion margins.
The first generation instrument was delivered to MSKCC in 2018 and has been used on over 250 patients to date. The several clinical papers published by the MSKCC collaborators have demonstrated the clear clinical potential of this technology to better manage patients affected by skin cancer lesions.
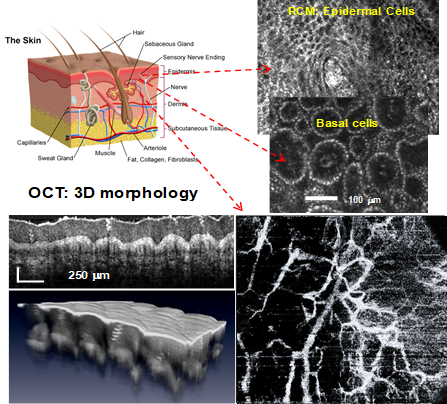
Skin morphology imaging with RCM-OCT enabling cellular level visualization, overall morphology assessment, and vascular changes
The second generation instrument provides improved resolution, imaging depth, and enables the analysis of skin vasculature. Global vascular patterns are helpful in the diagnosis of BCCs, as outlined by several authors. These patterns include either clustered (vessels with similar morphology closely gathered together), scattered (vessels with irregular and diffuse distribution), homogenous (densely aligned symmetrical vessels), or avascular (no vessels can be seen).
This probe will be used in a large study at MSKCC to guide radiotherapy on patients with BCCs on the head and neck area.
Multiphoton Microscopy Instrument for Assessing Small Animal Brain Neurodynamics
A team led by Dr. Youbo Zhao has developed the first generation neuroimager based on a two-photon microscopy (TPM) platform, which uses a fiber-based supercontinuum (SC) source to enable simultaneous excitation of multiple fluorophores and track the dynamics of the neurons. Team members include Gopi Maguluri, software development; John Grimble, mechanical design and fabrication; and Nicusor Iftimia, instrument architecture design.
The instrument was tested on both in vitro cultured rat primary neurons and in vivo brain of GCaMP6-expressing mice (a collection of ultrasensitive, green fluorescent indicator proteins that enable reliable detection of single action potential responses in vivo and facilitate the measurement of synaptic calcium signals).
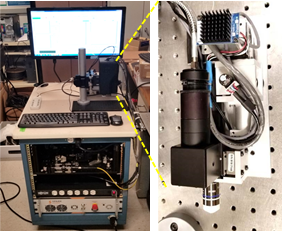
Representative data collected during in vivo imaging of a GCaMP6-expressing mouse brain are shown below. High resolution depth-scanned images demonstrate the high performance of the prototype instrument for in vivo brain imaging traces of Calcium dynamics from firing neurons (below) shows the high speed and high signal-to-noise-ratio (SNR) measurement of neuronal activities in the brain in vivo. To the best of our knowledge, this is the first time demonstration of in vivo brain imaging using a fiber-delivered SC source and a fiber-connected microscope.
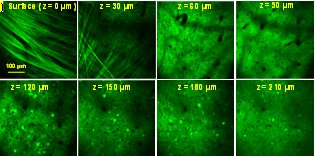
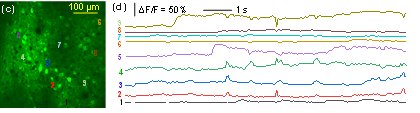
In vivo imaging of mouse brain expressing GCaMP6 (top) Depth-scanning images, (bottom left) still image of a cell layer at ~ 150 µm from the surface and (bottom right) spontaneous temporal Ca+ dynamics of selected neurons
This imaging tool will enable the assessment of drug delivery efficacy by monitoring the localization and quantitation of drug uptake by neuronal cells and the evaluation of its therapeutic effect by monitoring changes in the neural function of the brain.
PSI is seeking funding to support instrument improvement and further in vivo validation by experimental neuroscientists. All of these experiments will support the commercialization of the multi-excitation two-photon microscopy technology.
For more information, contact Dr. Nick Iftimia
Sensor Applications Group Highlights
Process Development Tools for Pharmaceutical Lyophilization
The Sensor Applications business area, led by Bill Kessler, recently completed a funded effort from the National Institute for Innovation Manufacturing Biopharmaceuticals (NIIMBL) to develop Software and Hardware Tools for Pharmaceutical Lyophilization Scale-up. The collaborative effort led by PSI, partnered with the University of Connecticut (UConn),
University of Massachusetts Lowell (UML), Purdue University, Genentech and Merck, focused on developing and demonstrating applications of Quality by Design (QbD) based freeze-drying process development tools to enable the generation of critical process knowledge and design space information to support lyophilization process engineering. The effort included the development of a new LyoBay, aseptic pilot scale lyophilization facility at UML, with funding support from the Massachusetts Life Sciences Center (MLSC), NIIMBL and UML. PSI supplied a LyoFlux® water vapor mass flow rate monitor to support process and design space development.
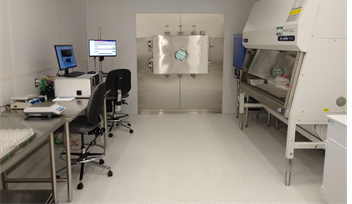
UML ISO-5 LyoBay clean room outfitted with pilot scale lyophilizer and LyoFlux®water vapor mass flow rate monitor
A third to a half of pharmaceuticals, particularly biotechnology products, do not have sufficient chemical and physical stability to withstand the rigors of distribution and storage in aqueous solution, so conversion to a stable solid is essential. Lyophilization, also known as freeze-drying, is a process by which a dry solid is produced from a solution, normally aqueous, by first converting most of the solvent to solid (ice), removing most of the water by sublimation, and finally, removing any unfrozen water by desorption. Freeze-drying is a complex and expensive process due to long process times. Commercial freeze-drying plants are very expensive to purchase and maintain. This motivates the development of efficient cycles that produce high quality drug product for all vials dried within the lyophilizer. Our R&D effort focused on developing new tools to support development of efficient freeze-drying processes and account for intra-batch drying heterogeneity.
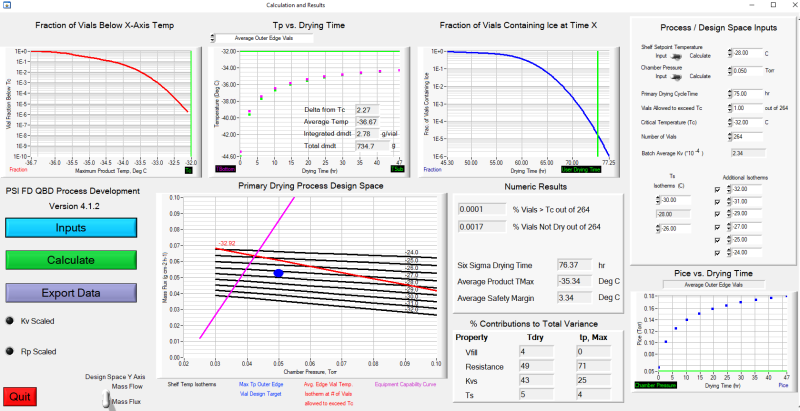
Heterogeneous freeze-drying model GUI
With support from UConn, PSI developed process development software implementing a heat and mass transfer model of lyophilization. Although numerous models have been developed by academia and industry, none accounted for drying heterogeneity in the process until researchers at UConn, led by Dr. Michael Pikal, developed a model. PSI worked with Dr. Robin Bogner at UConn to turn that model into user-friendly software, complete with a multi-screen graphical user interface (GUI) to support experimental data analysis and prediction of product temperatures and primary drying times as a function of vial location within the product-drying chamber of the lyophilizer. The software predictive capability was tested through a series of drying experiments at PSI, UConn and UML using both excipient formulations and drug substance supplied by our industry partners. This software is now being used at Genentech and Merck, and PSI is supporting process development for pharmaceutical clients.
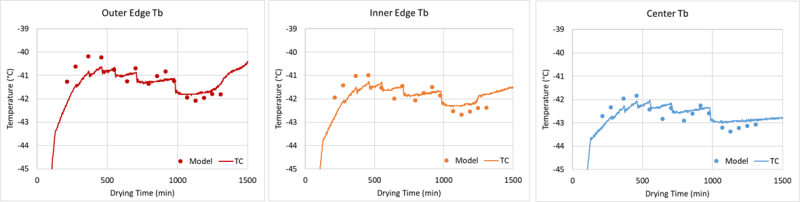
Comparison of model predicted and real-time product temperature measurements during drying
In addition to the process design software, Purdue University developed a compact model of vapor flow from the lyophilizer chamber to the condenser, enabling the prediction of the maximum mass flow rate curve for the dryer based on equipment capability. This model was verified by mass flow rate data and equipment capability measurements provided by the PSI LyoFlux® sensor. The equipment capability limits are a critical input to the heat and mass flow rate model described above. The development and testing of both of these models is documented in two peer-reviewed publications:
• “A Software Tool for Lyophilization Primary Drying Process Development and Scale-up Including Process Heterogeneity, I: Laboratory-Scale Model Testing”, PharmSciTech 2021 22(8) 274.
• “A Compact Model for Lyophilizer Equipment Capability Estimation”, PharmSciTech 2021 23(1) 14.
A follow-on effort, again led by PSI with participation from UConn, UML, SP Scientific, Genentech, Merck, and Pfizer, is leveraging the heterogeneous freeze-drying model and the LyoBay facility at UML. The goal of the Automated Process Control of Pharmaceutical Lyophilization: Process Analytical Technology (PAT) Enabled Freeze-Dryer R&D effort is to create process development and control software based on critical product parameters, real-time determination of product temperatures during primary drying, and to account for process drying heterogeneity. End users choose what PAT tools are available on their lyophilizer. A SMART algorithm, initially developed by UConn and Purdue, and commercialized by SP Scientific, is combined with the heterogeneous model of freeze-drying and real-time measurements to develop and control the drying process. PSI is leading the software algorithm development in collaboration with SP Scientific, with collaborative testing support provided by UConn and UML. Our pharmaceutical industry partners are providing scientific review and representative drug substances for software performance validation. The result of this effort will be a tool that develops and controls lyophilization cycles, accounting for intra- and inter-batch drying heterogeneity, resulting in a high quality product independent of vial location within the drying chamber.
Key contributors to both efforts at PSI include Emily Gong, Michael Hinds, Tiffany Yu and Bill Kessler.
For more information, contact Bill Kessler
Contract News
PSI recently received the following research contracts:
“Continuous Quantitative Methane Emission Monitor with Vent Discrimination” from the U.S. Environmental Protection Agency
“Compact Optical Fiber for Extreme Environments (COFFEE)” from the Defense Threat Reduction Agency
“Ultra High Temperature Large Format DMD-based IR Scene Projector” and “Radiation Hardened Foveated Hybrid Infrared Scene Projector” from the Missile Defense Agency
For more PSI news, visit www.psicorp.com/news.
Physical Sciences Inc. | contact@psicorp.com | (978) 689-0003
© 2022 Physical Sciences Inc.











