Newsletter
MassVentures announced the winners of $3.1 million in Small Business Innovative Research (SBIR) Targeted Technologies (START) grants that include PSI’s Imperia Batteries for specialty high energy lithium ion batteries and PSI’s IOScann – Intelligent Optical Scanner for guiding breast surgeries.
MassVentures provides equity financing to early stage startups and grant funding to SBIR/STTR Phase II funded entities located in Massachusetts. They reinvest the profits from their venture investments as an evergreen fund and align with entrepreneurs for long-term success. The START program helps growing Massachusetts-based companies convert research developed under SBIR and STTR contracts into businesses and jobs in Massachusetts. The START program plays an important role in powering Massachusetts’ Innovation Economy.
Imperia Batteries

PSI has received START Stage III funding to continue supporting the growth of a specialty battery production business based on our patented “High Active” (HA) coating technology. This technology can both improve the performance and reduce the cost of a lithium ion battery. Central to the value of the technology is the ability to apply the HA coating to various state-of-the-art cathode and anode materials employed in lithium ion batteries. The HA technology has been employed to create batteries that increase the operational endurance of unmanned vehicles for multiple applications. During Stage II, PSI established a manufacturing facility to produce batteries incorporating the HA technology for specialty applications under the product line Imperia Batteries (www.imperiabatteries.com). These batteries offer:
• Increased gravimetric and volumetric energy and power density, resulting in lighter and thinner batteries across a broad range of Li-ion battery chemistries.
• Reduced cost, environmental impact, and waste during production, enabling achievement of a cost-competitive price at lower production volumes.
The HA technology, developed using internal PSI funding and subsequently through a DOE Phase II program, has been brought from feasibility into initial production of batteries for targeted applications. A custom battery utilizing the HA technology was developed for PSI’s internal UAV program that delivers 47% greater energy density than the currently used commercial lithium ion battery and extended the operating duration of the InstantEye® UAV from ~30 minutes to 50-60 minutes.

Stage II START funding was essential to establishing initial battery production capability that led to production and delivery of the first batteries. This funding also supported performance of work under an electric vehicle technology demonstration effort that will clearly demonstrate the cost advantages of the HA technology. The START Stage III program will continue to extend our experience with the DoD to develop specialty batteries and associated marketing strategies for UAVs and UUVs.
For more information, contact Dr. Christopher Lang, Area Manager, Energy Technologies, at lang@psicorp.com.
IOScann
PSI has received START Stage I funding to continue supporting the growth of a medical product for guiding breast cancer surgeries. This product is called Intelligent Optical Scanner (IOScann) and it addresses an unmet clinical need for guidance of breast lumpectomy surgical procedures by assessing the surgical margins of the tissue removed, without the need for sample staining and preparation. The absence of cancer cells at the surgical margin indicates that no cancer cells were left in the surgical bed. Currently, surgical specimens are sent to the pathology lab and read for results days later, after the patient has gone home. If a positive margin is identified (presence of cancer at the outer edge of the specimen), there is a major risk for tumor recurrence, and the patient has to undergo a second surgery. This results in both financial costs and a tremendous emotional cost for both the patient and the family members.
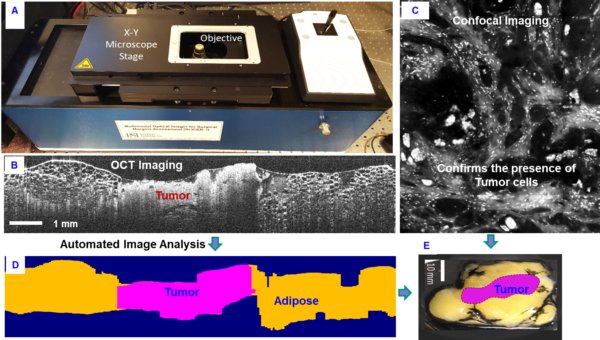
PSI’s IOScann generates real-time, high-resolution digital images of the tissue removed during surgery, and provides immediate feedback to the surgeon regarding the status of the surgical margin. The IOScann product uniquely combines three imaging modalities (fluorescence, RCM and OCT) that enable rapid assessment of the surgical specimens by highlighting areas that present positive surgical margins. Competitive measurement approaches such as Micro CT, Raman Spectroscopy and Fluorescence Imaging do not provide the resolution needed to differentiate positive margins, or are very slow to provide results making them unsuitable for intraoperative use. PSI’s IOScann is a revolutionary new product.
This capability was demonstrated during an NIH Phase II SBIR program by using a fluorescence guided, reflective confocal microscopy-optical coherence tomography (FG-RCM/OCT) imaging instrument. A sensitive contrast agent was developed and used to indicate positive margins and high-resolution imaging was used to precisely indicate the extent of the margins.
For more information, contact Dr. Nick Iftimia, Area Manager, Biomedical Optics Technology,at iftimia@psicorp.com
Antimicrobial Surface Coatings to Reduce COVID-19 Spread
PSI, in collaboration with National Emerging Infectious Disease Laboratories at Boston University (NEIDL/BU), has been awarded an SBIR Phase II contract from the U.S. Air Force to further develop PSI’s antimicrobial coating and demonstrate its effectiveness on an existing Patient Movement Item (PMI) litter product and other DoD medical equipment.
The Air Force has identified an urgent need to reduce COVID-19 contaminant loads in environments where Airmen operate (e.g., patient transfer mobility aircraft) and thus decrease transmission likelihood. PSI’s lightweight evacuation litter contains many materials representative of Air Force PMIs. PSI will coat these materials with a permanently attached, broad-spectrum antimicrobial technology. The coating efficacy will be demonstrated against a virus panel and other pathogens of interest. The coating will be optimized to achieve high levels of viral reduction within a short amount of time. The robustness of coating in litter operation will be evaluated upon weathering, abrasion, and cleaning. The application, efficacy, and robustness demonstrations will be extended to include other equipment that are potential disease vectors.
The PSI team will be solving the problem of reducing the transmission of COVID-19 and other infections, to help the Air Force Medical Service (AFMS) accomplish the goal of utilizing novel antimicrobial coating technologies to protect the Airmen in environments such as patient transfer mobility aircraft and other equipment. The technology will be applied to a wide range of equipment from surgical tools to evacuation aircraft.
For more information, contact Dr. Dorin Preda, Group Leader, Material Technologies at [dpreda@psicorp.com](mailto:The Air Force has identified an urgent need to reduce COVID-19 contaminant loads in environments where Airmen operate (e.g., patient transfer mobility aircraft) and thus decrease transmission likelihood. PSI’s lightweight evacuation litter contains many materials representative of Air Force PMIs. PSI will coat these materials with a permanently attached, broad-spectrum antimicrobial technology. The coating efficacy will be demonstrated against a virus panel and other pathogens of interest. The coating will be optimized to achieve high levels of viral reduction within a short amount of time. The robustness of coating in litter operation will be evaluated upon weathering, abrasion, and cleaning. The application, efficacy, and robustness demonstrations will be extended to include other equipment that are potential disease vectors. The PSI team will be solving the problem of reducing the transmission of COVID-19 and other infections, to help the Air Force Medical Service (AFMS) accomplish the goal of utilizing novel antimicrobial coating technologies to protect the Airmen in environments such as patient transfer mobility aircraft and other equipment. The technology will be applied to a wide range of equipment from surgical tools to evacuation aircraft. For more information, contact Dr. Dorin Preda, Group Leader, Material Technologies at dpreda@psicorp.com )
Contract News
PSI recently received the following research contracts:
“Multimodal Optical Probe for Skin Cancer Detection and Therapy Guidance” and “Ultra-sensitive Singlet Oxygen Dosimeter for Skin Cancer Treatment and Prevention” from the National Institutes of Health
“Ultra-compact Laser Ceilometer for Boundary Layer and Cloud Height Retrievals” from the Department of Energy
“Solar Concentrator System for Lunar ISRU Applications” from NASA
For more PSI news, visit www.psicorp.com/news



